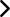Newsroom
Featured
Discover the Latest CMMS Insights and Industry News from PEMAC
This is the PEMAC resources section, your go-to destination for maintenance management market intelligence, thought leadership, and up-to-date information on CMMS software.
Our Latest News & Blogs

CMMS, LATEST NEWS, Life sciences, MAINTENANCE MANAGEMENT SOFTWARE, Manufacturing

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, Manufacturing, PEMAC ASSETS
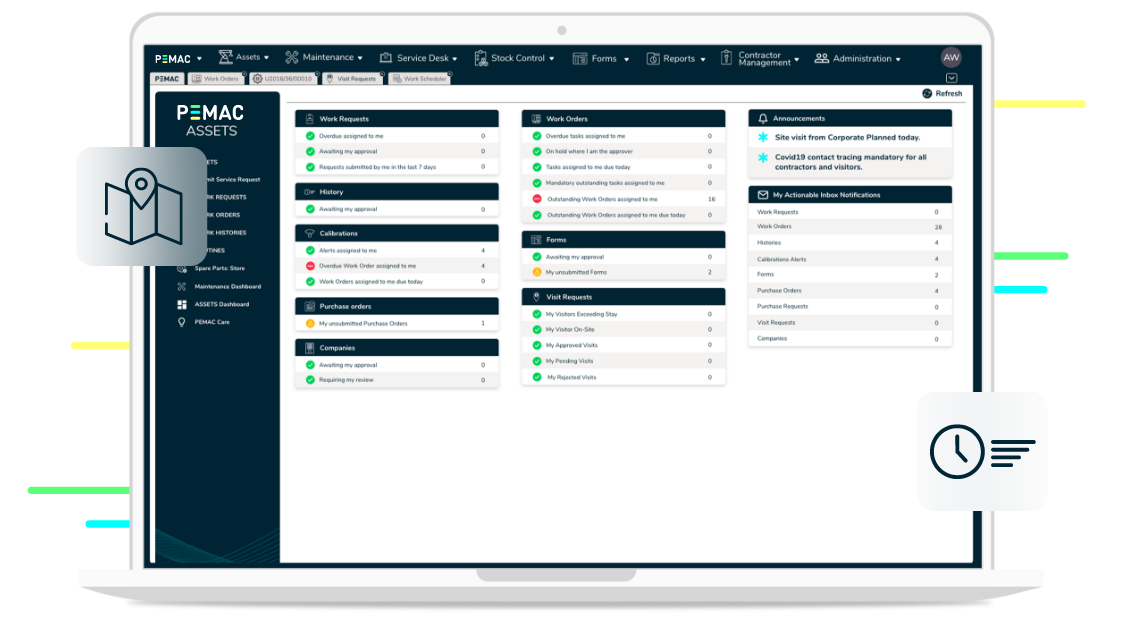
CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE

LATEST NEWS, Opening Hours

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, Manufacturing, PEMAC ASSETS

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, Manufacturing, PEMAC ASSETS

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, PEMAC ASSETS, Training

LATEST NEWS, Opening Hours

CMMS, LATEST NEWS, Life sciences, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, LATEST NEWS, Life sciences, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, PEMAC ASSETS, Training

LATEST NEWS, Opening Hours
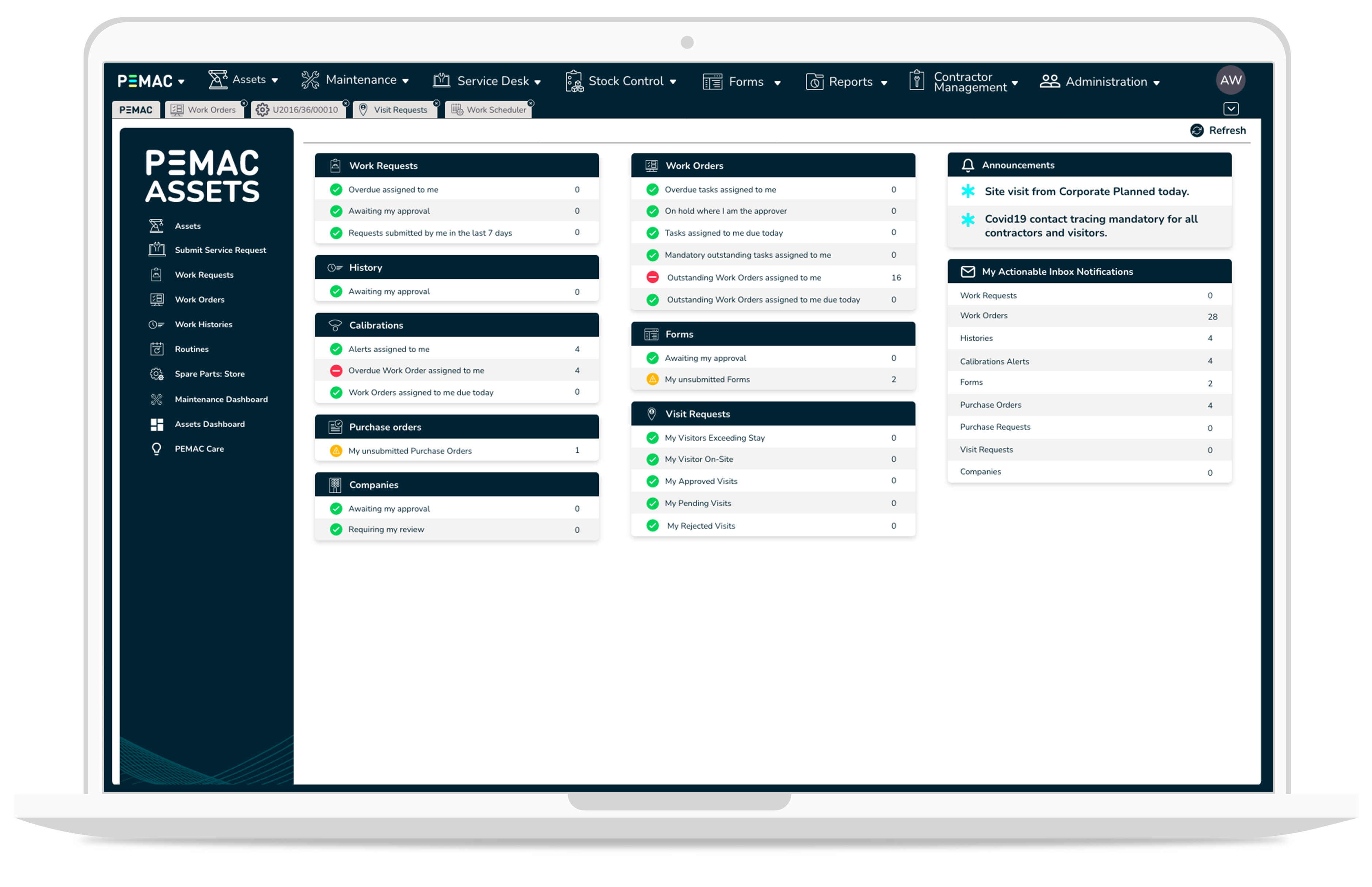
CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, MAINTENANCE MANAGEMENT SOFTWARE, Webinar
CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, ESG, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, Sustainability

CMMS, ERP, LATEST NEWS, Systems Integration
CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE
CMMS, LATEST NEWS, New Software Release, PEMAC ASSETS

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, Uncategorised
Asset Performance Management, CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE, Uncategorised

CMMS, LATEST NEWS, MAINTENANCE MANAGEMENT SOFTWARE